NEWPORT BEACH, CA – Building on an established track record of organic growth, Cure Medical recently announced that their full line of ready-to-use, 16-inch Cure hydrophilic catheters are now approved for sale in Canada.
 “The demand in Canada for Cure Medical catheters is exponentially increasing due to Canadian sales partners and end users who are looking for a quality-made, affordable intermittent catheter that is not made with known carcinogens like DEHP or BPA. We are thrilled to now provide our full line of hydrophilic catheters for men and women in Canada. Cure Medical will continue to expand our product availability and brand awareness in this market,” Cure Medical CEO John Anderson says.
“The demand in Canada for Cure Medical catheters is exponentially increasing due to Canadian sales partners and end users who are looking for a quality-made, affordable intermittent catheter that is not made with known carcinogens like DEHP or BPA. We are thrilled to now provide our full line of hydrophilic catheters for men and women in Canada. Cure Medical will continue to expand our product availability and brand awareness in this market,” Cure Medical CEO John Anderson says.
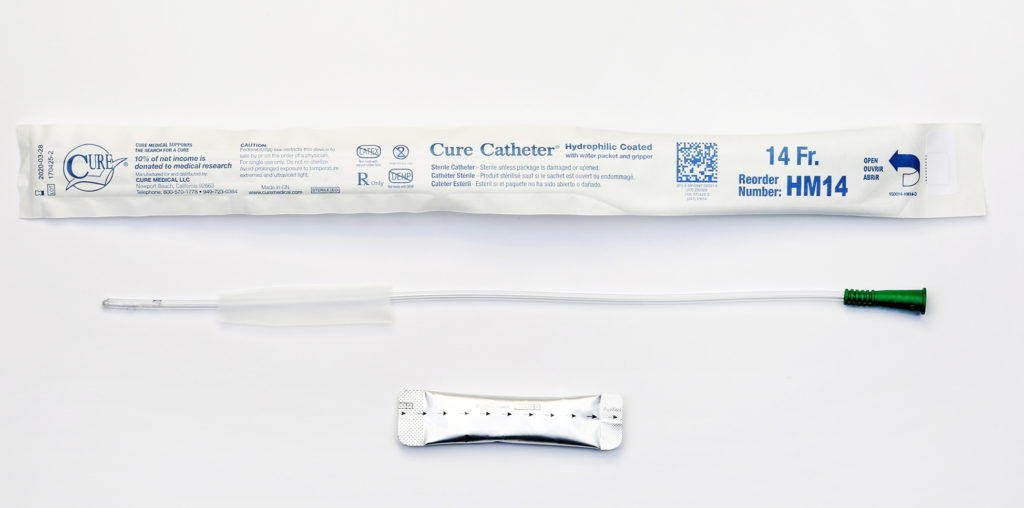
Cure Medical offers the only sterile, single-use hydrophilic catheter on the market that features all of these benefits:
- Smooth polished eyelets for increased comfort
- Straight or coude tip
- Does not kink when bent
- Available in French sizes 12-16
- Hydrophilic coating for quick lubrication
- Purified water packet for easy, mess-free/stain-free hydration
- Textured advancer/gripper for clean, easy catheter insertion
- High quality materials, not made with scary chemicals like DEHP*, BPA or natural rubber latex
Learn more about the concerns with DEHP here: https://curemedical.com/understanding-dehp-health/
“We also remain distinguished as the only intermittent catheter manufacturer in the world that donates 10% of all net income to fund medical research in pursuit of a cure for paralysis and central nervous system disorders,” Anderson adds. See Cure Medical at Medtrade, scheduled for Oct 15-17, 2018, at the Georgia World Congress Center in Atlanta.

